Why We Balance Chemical Equations
Understanding why we balance equations helps you organize your thinking efficiently and makes learning faster and more memorable. The two big reasons for balancing equations. First is so that they obey the Law of Conservation of Mass. Second is so we can use the coefficients as ratios between products and reactants.
Strategies for Success:
- Balancing equations means that the number of each type of atom is the same on both sides of the equation. The equation then obeys the Law of Conservation of Mass.
- We often use the coefficients in balanced equations as ratios (sometimes called mole ratios).
- Balanced equations are also used in chemistry topics like thermodynamics and stoichiometry.
Key Information
When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.
You can only change the coefficients when balancing!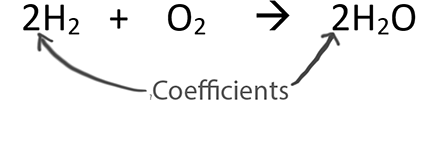
Balanced equations provide ratios between substances. The coefficients can be thought of as mole ratios.