How to Balance:
Li + O2 → Li2O
Word equation: Lithium + Oxygen gas → Lithium oxide
Type of Chemical Reaction: For this reaction we have a combination reaction.
Balancing Strategies: In this combination reaction we have the lithium coming together with the oxygen gas to form within oxide.
To balance the equation you first need to get the oxygen atoms to be equal on both sides of the equation.
Essential Resources
For a complete explanation, watch:
When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.
Only change the coefficients (these are the numbers in front substances).
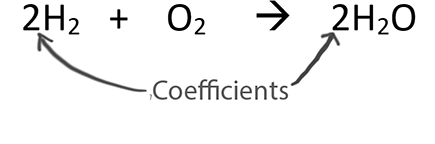
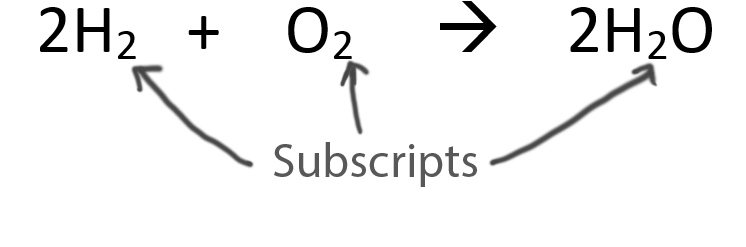
Video:
Subscribe