How to Balance:
CaCl2 + Na3PO4 → Ca3(PO4)2 + NaCl
Word equation: Calcium chloride + Sodium phosphate → Calcium phosphate + Sodium chloride
Type of Chemical Reaction: For this reaction we have a double replacement reaction.
Balancing Strategies: This is a bit of a challenging chemical reaction to balance. It is helpful if you consider the SO4 (sulfate) to be one thing since it is on both sides of the equation. Same for the hydroxide (OH).This will make the counting much easier.
If you stuck balancing this equation, watch the video for more help.
Essential Resources
For a complete explanation, watch:
When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.
Only change the coefficients (these are the numbers in front substances).
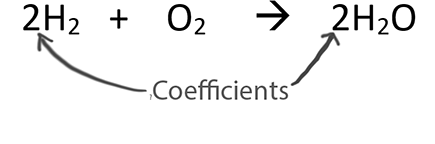
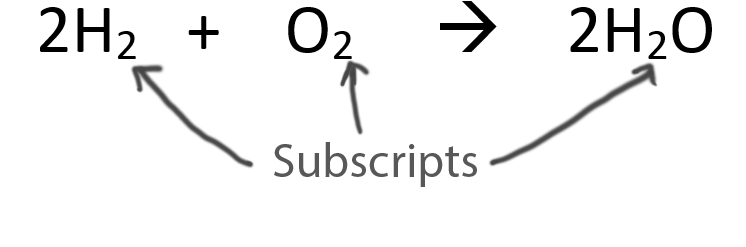
Video:
Subscribe