How to Balance:
HBr + Fe(OH)3 → FeBr3 + H2O
Word equation: Hydrobromic acid + Iron (III) hydroxide → Iron (III) bromide + Water
Type of Chemical Reaction: For this reaction we have a neutralization reaction.
Balancing Strategies: This is a neutralization reaction because the HBr and Fe(OH)3 are combining to form a salt, that's the FeBr3, and water.
We could also consider it a double and and dislacement reaction, and as well.
This is a little more challenging equation to balance so stick with it!
Essential Resources
For a complete explanation, watch:
When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.
Only change the coefficients (these are the numbers in front substances).
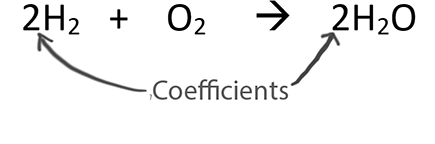
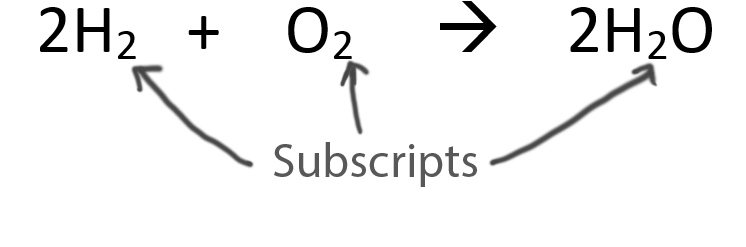
Video:
Subscribe